MENU
DK | DKK
DK | DKK
-
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- High-Speed and Ultracentrifugation Consumables
- Accessories
- Tubes
- Plates
- Device Management Software
- Sample and Information Management
- IVD Products
No results found
Search Suggestions

Stem Cell Cultivation in Bioreactors: Increasing hiPSC Yield
Robert Zweigerdt Lab Academy
- Bioprocessing
- Stem Cells
- Essay
Robert Zweigerdt from Hannover Medical School explains, how his team greatly increased the hiPSC yield in bioreactors.
Human induced pluripotent stem cells (hiPSCs) and their differentiated progeny provide the tool set for innovative approaches in regenerative medicine. Stirred-tank bioreactors are promising cultivation systems, as they have the capacity to produce high cell numbers, allowing scale-up and cutting-edge opportunities for improving the control of growth parameters, with numerous benefits, the foremost of which is improved cultivation reproducibility.
Increasing cell density is a vital component of research in stem cell bioprocessing. In this interview, Dr. Robert Zweigerdt from Hannover Medical School, Germany explains, how his team reached a culture yield of 35 million hiPSCs per mL.
Eppendorf: Your group has established expertise in cultivating hiPSCs as cell aggregates in stirred-tank bioreactors. In your latest publication, you reported attaining a cell density of 35 million cells per mL. That is a big leap! Which major hurdles did you have to overcome to reach this milestone?
RZ: The first hurdle, which we attacked a decade ago, was to support survival and proliferation of hiPSC seeded in a three dimensional (3D) matrix-free suspension culture, in contrast to the established cultivation protocols employing 2D matrix-dependent monolayer culture on conventional culture dishes and platforms 1, 2 .
The second big step, accomplished in collaboration with your company, was the design of a modified stirring impeller design supporting a more homogeneous hiPSC aggregation3 and, subsequently, a “retention-filter” system. Such retention systems enable keeping the hPS cells, which form multicellular aggregates in stirred suspension culture, in the bioreactor upon automated perfusion feeding, defined as constant replacement of used by fresh media4 . Subsequently, perfusion feeding was the prerequisite for our latest step: that is, the identification of growth-limiting parameters such as pH dependence, glucose consumption and lactate accumulation. Having identified these growth-limiting bottlenecks, feedback-based monitoring was performed which requires the control of the overall medium throughput via perfusion feeding. Dr. Felix Manstein, of our department, who was driving these investigations in recent years, has also implemented in silico process modeling and optimization strategies, which facilitate rational process development of high density bioprocessing of hiPSCs5 .
Increasing cell density is a vital component of research in stem cell bioprocessing. In this interview, Dr. Robert Zweigerdt from Hannover Medical School, Germany explains, how his team reached a culture yield of 35 million hiPSCs per mL.
Eppendorf: Your group has established expertise in cultivating hiPSCs as cell aggregates in stirred-tank bioreactors. In your latest publication, you reported attaining a cell density of 35 million cells per mL. That is a big leap! Which major hurdles did you have to overcome to reach this milestone?
RZ: The first hurdle, which we attacked a decade ago, was to support survival and proliferation of hiPSC seeded in a three dimensional (3D) matrix-free suspension culture, in contrast to the established cultivation protocols employing 2D matrix-dependent monolayer culture on conventional culture dishes and platforms 1, 2 .
The second big step, accomplished in collaboration with your company, was the design of a modified stirring impeller design supporting a more homogeneous hiPSC aggregation3 and, subsequently, a “retention-filter” system. Such retention systems enable keeping the hPS cells, which form multicellular aggregates in stirred suspension culture, in the bioreactor upon automated perfusion feeding, defined as constant replacement of used by fresh media4 . Subsequently, perfusion feeding was the prerequisite for our latest step: that is, the identification of growth-limiting parameters such as pH dependence, glucose consumption and lactate accumulation. Having identified these growth-limiting bottlenecks, feedback-based monitoring was performed which requires the control of the overall medium throughput via perfusion feeding. Dr. Felix Manstein, of our department, who was driving these investigations in recent years, has also implemented in silico process modeling and optimization strategies, which facilitate rational process development of high density bioprocessing of hiPSCs5 .
Read more
Read less

BioBLU 0.3 sc Single-Use Bioreactor equipped with 8-blade impeller
Eppendorf: For a prospective use in advanced therapies, hiPSCs need to be differentiated into the desired cell type. How straightforward was it to translate differentiation protocols which have been designed for monolayer cultures to cell aggregates in bioreactors?
RZ: Since we initiated the development of lineage-specific differentiation strategies in suspension several years ago, shortly after the first successful hiPSC culture in 3D6 , we have established a substantial degree of competency in that area as well. The most significant challenges regarding directed differentiation in suspension culture include the impact of cell aggregates size, its heterogeneity, overall cell density and defining mechanical and hydrodynamic parameters7 .
However, we also noted that the standard culture media components and differentiation-directing molecules that we are applying, as for example the WNT pathway modulators, used for mesendoderm-induction and cardiac differentiation, have equivalent effects in 2D and in 3D8 . Therefore, process transition from 2D, which is often applied for cell differentiation basic research, to 3D suspension culture is typically straightforward. However, we are convinced that in the future many differentiation strategies will benefit from advance process control abilities enabled by the bioreactor technologies, still in the early stages of development9 .
Notably, we demonstrated that stirred-tank bioreactor-based hiPSC differentiation is efficiently applicable not only for cardiac diffraction (as highlighted by references above) but also for the differentiation and production of numerous other functional hiPSC progenies, including endothelial cells10 macrophages11 and endodermal derivatives12 .
Eppendorf: In upstream bioprocessing, the feeding strategy strongly impacts cell growth and viability. Repeated batch and perfusion are two options for removing byproducts and replenishing nutrients. What do you consider the pros and cons of these two strategies?
RZ: As mentioned above, our experience suggests that perfusion feeding, despite its complexity, is the optimal tactic for advanced hPSC cultivation13 . This is due to the highly glycolytic metabolism of the rapidly growing hPSC, which, on the one hand, requires an enormous supply of extra glucose to avoid growth-limiting starvation. Moreover, on the other hand, high glucose supplementation results into a massive accumulation of secreted lactate, which may become toxic and which induces a proliferation-inhibiting acidification of the culture. These issues increase exponentially in parallel to the exponential increase in cell density5 . For these reasons, we feel that perfusion feeding is the most successfully strategy to control growth-limiting parameters, if the goal of the protocol is to optimize high density cultivation of hiPSC. Notably, in parallel to the 10-fold increase in cell density, the amount of medium required to generate a given number of cells, was reduced by 70 % in consequence to the process optimization steps.
RZ: Since we initiated the development of lineage-specific differentiation strategies in suspension several years ago, shortly after the first successful hiPSC culture in 3D6 , we have established a substantial degree of competency in that area as well. The most significant challenges regarding directed differentiation in suspension culture include the impact of cell aggregates size, its heterogeneity, overall cell density and defining mechanical and hydrodynamic parameters7 .
However, we also noted that the standard culture media components and differentiation-directing molecules that we are applying, as for example the WNT pathway modulators, used for mesendoderm-induction and cardiac differentiation, have equivalent effects in 2D and in 3D8 . Therefore, process transition from 2D, which is often applied for cell differentiation basic research, to 3D suspension culture is typically straightforward. However, we are convinced that in the future many differentiation strategies will benefit from advance process control abilities enabled by the bioreactor technologies, still in the early stages of development9 .
Notably, we demonstrated that stirred-tank bioreactor-based hiPSC differentiation is efficiently applicable not only for cardiac diffraction (as highlighted by references above) but also for the differentiation and production of numerous other functional hiPSC progenies, including endothelial cells10 macrophages11 and endodermal derivatives12 .
Eppendorf: In upstream bioprocessing, the feeding strategy strongly impacts cell growth and viability. Repeated batch and perfusion are two options for removing byproducts and replenishing nutrients. What do you consider the pros and cons of these two strategies?
RZ: As mentioned above, our experience suggests that perfusion feeding, despite its complexity, is the optimal tactic for advanced hPSC cultivation13 . This is due to the highly glycolytic metabolism of the rapidly growing hPSC, which, on the one hand, requires an enormous supply of extra glucose to avoid growth-limiting starvation. Moreover, on the other hand, high glucose supplementation results into a massive accumulation of secreted lactate, which may become toxic and which induces a proliferation-inhibiting acidification of the culture. These issues increase exponentially in parallel to the exponential increase in cell density5 . For these reasons, we feel that perfusion feeding is the most successfully strategy to control growth-limiting parameters, if the goal of the protocol is to optimize high density cultivation of hiPSC. Notably, in parallel to the 10-fold increase in cell density, the amount of medium required to generate a given number of cells, was reduced by 70 % in consequence to the process optimization steps.
Read more
Read less
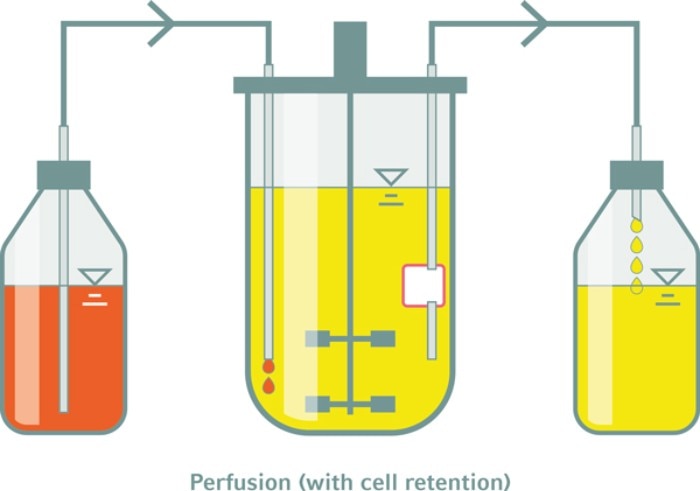
Perfusion feeding enables control of growth-limiting parameters
Eppendorf: Within a few years, you were able to increase hiPSC culture density more than tenfold. How did you optimize your process to obtain this value?
RZ: A couple of years back, we gained 2.85 million hiPSCs per mL following inoculation with 0.5 million cells per mL. Recently, we obtained a more than 10-fold higher cell density following a comparable inoculation density. We follow a step-by-step strategy, systematically analyzing the challenges and then applying the bioreactor-enabled combined control of the parameters, thereby overcoming the growth-limiting hurdles. Specific bottlenecks include: promoting efficient survival and aggregation of hiPSC after single cell-based process inoculation, appropriate adaptation of the stirring speed to ensure no hiPSC-clumping and reducing aggregate diameter below ~300 µm. Next, avoiding pH drop below circa 6.7, ensuring constant glucose supply to avoid cell starvation and adapting the perfusion speed (and promoting thus the optimal medium throughput) to avoid peak accumulation of lactate and toxic osmolality levels as well as several additional parameters5 . However, once these limitations are identified, they can be systematically controlled via the bioprocess control software, and optimized in combination with in silico process modeling; details in our most recent protocol14 .
RZ: A couple of years back, we gained 2.85 million hiPSCs per mL following inoculation with 0.5 million cells per mL. Recently, we obtained a more than 10-fold higher cell density following a comparable inoculation density. We follow a step-by-step strategy, systematically analyzing the challenges and then applying the bioreactor-enabled combined control of the parameters, thereby overcoming the growth-limiting hurdles. Specific bottlenecks include: promoting efficient survival and aggregation of hiPSC after single cell-based process inoculation, appropriate adaptation of the stirring speed to ensure no hiPSC-clumping and reducing aggregate diameter below ~300 µm. Next, avoiding pH drop below circa 6.7, ensuring constant glucose supply to avoid cell starvation and adapting the perfusion speed (and promoting thus the optimal medium throughput) to avoid peak accumulation of lactate and toxic osmolality levels as well as several additional parameters5 . However, once these limitations are identified, they can be systematically controlled via the bioprocess control software, and optimized in combination with in silico process modeling; details in our most recent protocol14 .
Read more
Read less
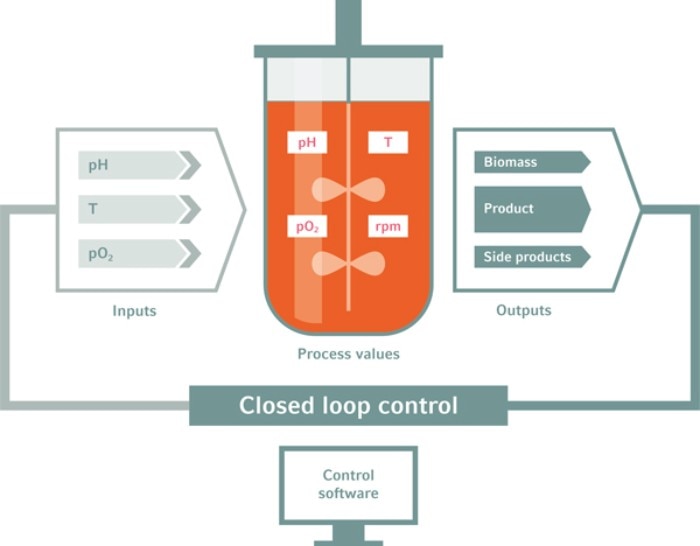
In bioreactors, process parameters can be closely controlled
Eppendorf: You were able to generate 5.25 billion hiPSCs in a 150 mL volume. How does that number compare to the number of cells required for cell therapy applications, for example for the heart? Do you see the need for future scale-up?
RZ: Despite this substantial progress of bioprocessing of undifferentiated, pluripotent hiPSCs, we are still working on further increasing the cell density and thus the yield of differentiated cells including hiPSC-derived cardiomyocytes. While we have achieved very high lineage purity e.g. of >95% iPSC-cardiomyocytes, the cell density obtained from the differentiation protocol is still relatively low; currently ca. 1-2x106 cells per mL8 . Since estimations suggest that for the replacement of disease-depleted heart myocytes about 1-2x109 iPSC-cardiomyocytes will be required for each patient, we would currently require about a 1 liter culture to provide the appropriate cell dose for an individual patient. Regenerative medicine researchers are discussing the possibility of generating very large cell batches for an allogeneic – non-patient specific – transplantation approach, so we believe it would be appropriate to pursue a program of substantial upscaling in the future. This goal would target volumes of five, ten, twenty, one hundred-fold and eventually even greater levels.
Such upscaling strategy is also highly attractive from a commercial perspective, which includes transition to fully controlled GMP-conditions required for regulatory compliance and clinical translation.
Another promising approach is “blood cell farming” as for example the differentiation of functional macrophages from hiPSC. As we recently demonstrated in collaboration with the group of Ncio Lachmann at the Hannover Medical School campus, this approach, in contrast to the batch-production of hiPSC-cardiomyocytes, is compatible with the continuing production of macrophages in stirred tank bioreactors over several weeks or even months11 .
Eppendorf: Considering the cell density, do you still see room for improvement? Which are the limiting factors?
RZ: As indicated above, we see room for improvement for the bioprocessing of differentiated hiPSC progenies. The (limiting) factors for differentiation are even more complex compared to the expansion of hiPSC at the pluripotent state. The reasons for this include the higher complexity of differentiation processes since the cells are constantly changing their progenitor status and phenotype and thus their physiology and proliferation properties. We are working intensively to develop lineage-specific process conditions expressing numerous different lineages.
It is a challenging task but therefore inspiring and exciting!
RZ: Despite this substantial progress of bioprocessing of undifferentiated, pluripotent hiPSCs, we are still working on further increasing the cell density and thus the yield of differentiated cells including hiPSC-derived cardiomyocytes. While we have achieved very high lineage purity e.g. of >95% iPSC-cardiomyocytes, the cell density obtained from the differentiation protocol is still relatively low; currently ca. 1-2x106 cells per mL8 . Since estimations suggest that for the replacement of disease-depleted heart myocytes about 1-2x109 iPSC-cardiomyocytes will be required for each patient, we would currently require about a 1 liter culture to provide the appropriate cell dose for an individual patient. Regenerative medicine researchers are discussing the possibility of generating very large cell batches for an allogeneic – non-patient specific – transplantation approach, so we believe it would be appropriate to pursue a program of substantial upscaling in the future. This goal would target volumes of five, ten, twenty, one hundred-fold and eventually even greater levels.
Such upscaling strategy is also highly attractive from a commercial perspective, which includes transition to fully controlled GMP-conditions required for regulatory compliance and clinical translation.
Another promising approach is “blood cell farming” as for example the differentiation of functional macrophages from hiPSC. As we recently demonstrated in collaboration with the group of Ncio Lachmann at the Hannover Medical School campus, this approach, in contrast to the batch-production of hiPSC-cardiomyocytes, is compatible with the continuing production of macrophages in stirred tank bioreactors over several weeks or even months11 .
Eppendorf: Considering the cell density, do you still see room for improvement? Which are the limiting factors?
RZ: As indicated above, we see room for improvement for the bioprocessing of differentiated hiPSC progenies. The (limiting) factors for differentiation are even more complex compared to the expansion of hiPSC at the pluripotent state. The reasons for this include the higher complexity of differentiation processes since the cells are constantly changing their progenitor status and phenotype and thus their physiology and proliferation properties. We are working intensively to develop lineage-specific process conditions expressing numerous different lineages.
It is a challenging task but therefore inspiring and exciting!
Read more
Read less
References:
[1] Up-scaling single cell-inoculated suspension culture of human embryonic stem cells. Singh H, et al. Stem Cell Res. 2010 May;4(3):165-79. doi: 10.1016/j.scr.2010.03.001. Epub 2010 Mar 12.
[2] Scalable expansion of human pluripotent stem cells in suspension culture. Zweigerdt R, et al. Nat Protoc. 2011 May;6(5):689-700. doi: 10.1038/nprot.2011.318. Epub 2011 Apr 28.
[3] Suspension culture of human pluripotent stem cells in controlled, stirred bioreactors. Olmer R, et al. Tissue Eng Part C Methods. 2012 Oct;18(10):772-84. doi: 10.1089/ten.TEC.2011.0717. Epub 2012 Jun 4.
[4] Impact of Feeding Strategies on the Scalable Expansion of Human Pluripotent Stem Cells in Single-Use Stirred Tank Bioreactors. Kropp C, et al. Stem Cells Transl Med. 2016 Oct;5(10):1289-1301. doi: 10.5966/sctm.2015-0253. Epub 2016 Jul 1.
[5] High density bioprocessing of human pluripotent stem cells by metabolic control and in silico modeling. Manstein F, et al. Stem Cells Transl Med. 2021 Jul;10(7):1063-1080. doi: 10.1002/sctm.20-0453. Epub 2021 Mar 4.
[6] Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Kempf H, et al. Stem Cell Reports. 2014 Dec 9;3(6):1132-46. doi: 10.1016/j.stemcr.2014.09.017. Epub 2014 Oct 30.
[7] Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Kempf H, et al. Nat Protoc. 2015 Sep;10(9):1345-61. doi: 10.1038/nprot.2015.089. Epub 2015 Aug 13.
[8] Continuous WNT Control Enables Advanced hPSC Cardiac Processing and Prognostic Surface Marker Identification in Chemically Defined Suspension Culture. Halloin C, et al. Stem Cell Reports. 2019 Aug 13;13(2):366-379. doi: 10.1016/j.stemcr.2019.06.004. Epub 2019 Jul 25.
[9] Prediction of Human Induced Pluripotent Stem Cell Cardiac Differentiation Outcome by Multifactorial Process Modeling. Williams B, et al. Front Bioeng Biotechnol. 2020 Jul 23;8:851. doi: 10.3389/fbioe.2020.00851. eCollection 2020.
[10] Differentiation of Human Pluripotent Stem Cells into Functional Endothelial Cells in Scalable Suspension Culture. Olmer R, et al. Stem Cell Reports. 2018 May 8;10(5):1657-1672. doi: 10.1016/j.stemcr.2018.03.017. Epub 2018 Apr 19.
[11] Bioreactor-based mass production of human iPSC-derived macrophages enables immunotherapies against bacterial airway infections. Ackermann M, et al. Nat Commun. 2018 Nov 30;9(1):5088. doi: 10.1038/s41467-018-07570-7.
[12] Chemically-Defined, Xeno-Free, Scalable Production of hPSC-Derived Definitive Endoderm Aggregates with Multi-Lineage Differentiation Potential. Sahabian A, et al. Cells. 2019 Dec 4;8(12):1571. doi: 10.3390/cells8121571.
[13] Progress and challenges in large-scale expansion of human pluripotent stem cells Christina Kropp C, et al. Process Biochemistry Volume 59, Part B, August 2017, Pages 244-254
[14] Process control and in silico modelling strategies for enabling high density culture of human pluripotent stem cells in stirred tank bioreactors. Manstein F, et al. STAR Protocols, 2021 Dec 9;2(4):100988. doi: 10.1016/j.xpro.2021.100988. eCollection 2021 Dec 17.
[1] Up-scaling single cell-inoculated suspension culture of human embryonic stem cells. Singh H, et al. Stem Cell Res. 2010 May;4(3):165-79. doi: 10.1016/j.scr.2010.03.001. Epub 2010 Mar 12.
[2] Scalable expansion of human pluripotent stem cells in suspension culture. Zweigerdt R, et al. Nat Protoc. 2011 May;6(5):689-700. doi: 10.1038/nprot.2011.318. Epub 2011 Apr 28.
[3] Suspension culture of human pluripotent stem cells in controlled, stirred bioreactors. Olmer R, et al. Tissue Eng Part C Methods. 2012 Oct;18(10):772-84. doi: 10.1089/ten.TEC.2011.0717. Epub 2012 Jun 4.
[4] Impact of Feeding Strategies on the Scalable Expansion of Human Pluripotent Stem Cells in Single-Use Stirred Tank Bioreactors. Kropp C, et al. Stem Cells Transl Med. 2016 Oct;5(10):1289-1301. doi: 10.5966/sctm.2015-0253. Epub 2016 Jul 1.
[5] High density bioprocessing of human pluripotent stem cells by metabolic control and in silico modeling. Manstein F, et al. Stem Cells Transl Med. 2021 Jul;10(7):1063-1080. doi: 10.1002/sctm.20-0453. Epub 2021 Mar 4.
[6] Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Kempf H, et al. Stem Cell Reports. 2014 Dec 9;3(6):1132-46. doi: 10.1016/j.stemcr.2014.09.017. Epub 2014 Oct 30.
[7] Cardiac differentiation of human pluripotent stem cells in scalable suspension culture. Kempf H, et al. Nat Protoc. 2015 Sep;10(9):1345-61. doi: 10.1038/nprot.2015.089. Epub 2015 Aug 13.
[8] Continuous WNT Control Enables Advanced hPSC Cardiac Processing and Prognostic Surface Marker Identification in Chemically Defined Suspension Culture. Halloin C, et al. Stem Cell Reports. 2019 Aug 13;13(2):366-379. doi: 10.1016/j.stemcr.2019.06.004. Epub 2019 Jul 25.
[9] Prediction of Human Induced Pluripotent Stem Cell Cardiac Differentiation Outcome by Multifactorial Process Modeling. Williams B, et al. Front Bioeng Biotechnol. 2020 Jul 23;8:851. doi: 10.3389/fbioe.2020.00851. eCollection 2020.
[10] Differentiation of Human Pluripotent Stem Cells into Functional Endothelial Cells in Scalable Suspension Culture. Olmer R, et al. Stem Cell Reports. 2018 May 8;10(5):1657-1672. doi: 10.1016/j.stemcr.2018.03.017. Epub 2018 Apr 19.
[11] Bioreactor-based mass production of human iPSC-derived macrophages enables immunotherapies against bacterial airway infections. Ackermann M, et al. Nat Commun. 2018 Nov 30;9(1):5088. doi: 10.1038/s41467-018-07570-7.
[12] Chemically-Defined, Xeno-Free, Scalable Production of hPSC-Derived Definitive Endoderm Aggregates with Multi-Lineage Differentiation Potential. Sahabian A, et al. Cells. 2019 Dec 4;8(12):1571. doi: 10.3390/cells8121571.
[13] Progress and challenges in large-scale expansion of human pluripotent stem cells Christina Kropp C, et al. Process Biochemistry Volume 59, Part B, August 2017, Pages 244-254
[14] Process control and in silico modelling strategies for enabling high density culture of human pluripotent stem cells in stirred tank bioreactors. Manstein F, et al. STAR Protocols, 2021 Dec 9;2(4):100988. doi: 10.1016/j.xpro.2021.100988. eCollection 2021 Dec 17.
Read more
Read less
Related links:
Read more
Read less
